pigment composition


A pigment’s physical and chemical properties play a vital role in how it performs. Each inorganic (mineral or Earth) pigment has a unique elemental (chemical) composition in which elements such as iron or copper bond at the molecular level with other elements to create unique chemical compositions that influence everything about each pigment.
For example, the most common form of red iron oxide (red ochre) is composed of iron and oxygen: Fe₂O₃. But compare that to magnetite, which is another form of iron oxide: Fe₃O₄. This means plain old rust is composed of 2 parts iron and 3 parts oxygen, while magnetite is composed of 3 parts iron and 4 parts oxygen. While this may seem inconsequential, that slight compositional difference gives us two different materials with their own distinct color and properties.
Knowing a pigment’s elemental composition aids in conservation of artworks and gives us the ability to date a piece, determine authenticity, and lets us better understand the behaviors, lightfastness, durability, our paintings.
Knowing a pigment’s elemental composition aids in conservation of artworks and gives us the ability to date a piece, determine authenticity, and lets us better understand the behaviors, lightfastness, durability,compatibility with other pigments and much more. Each pigment’s opacity, tinting strength, lightfastness and even drying time are all due to its elemental composition. It also gives us information about how a pigment will perform with different media like oil, watercolor, egg yolk and more.
As we see with the red ochre and magnetite, even slight chemical variations affect the color, hardness, etc. It also affects the toxicity of certain pigments. Cadmium, lead, mercury, chromium and arsenic are just a few of the elements that determine toxicity. However, whether a pigment is toxic or not, it’s always advisable to wear a face mask and goggles when working with dry pigments (click here for more on safety and health).
Pigment chemistry also helps us understand how particular pigments degrade or react to environmental influences. It can also influence and make our pigment choices more intentional and improve the quality of our paintings. In the images below, the one on the left is the original painting; the one on the right is after the loss of red tones due to the degradation of the red pigments (mostly madder lake) he used to create the soft violet and purple colors.
"The Bedroom". Van Gogh, 1888. Left is original coloring, right is after loss of reds.
Knowing a pigment's elemental composition is crucial for art conservation (dating, authenticity, preservation), scientific understanding (chemical behavior, lightfastness), and environmental/health safety (toxicity, sustainability), as the elements dictate its color, stability, origin, and potential hazards, influencing everything from how an artist uses it to how it degrades over centuries.
For Art & History:
Dating & Provenance: Specific elements (like lead, mercury) or mineral compositions (like lapis lazuli) pinpoint when and where a pigment was available, helping date artworks or verify origin.
Authenticity: Detecting anachronistic elements (e.g., titanium white in a 17th-century painting) reveals forgeries or later alterations.
Conservation: Understanding the elements helps conservators predict reactions (e.g., tarnishing, fading) and choose appropriate cleaning/storage conditions to prevent damage.
For Artists & Material Science:
Performance: The chemistry dictates properties like opacity, tinting strength, drying time, and lightfastness, informing how pigments behave in different media (oil, watercolor, acrylic).
Color Variation: Different elemental arrangements (e.g., iron's oxidation states) create diverse colors from the same metal, explaining color shifts.
Technique: Knowing the chemical makeup helps artists select pigments for specific effects, like granulation or stability in washes.
For Health & Environment:
Toxicity: Many vibrant historical pigments (cadmium, lead, chromium) contain toxic heavy metals, posing risks during creation, use, and disposal, driving the development of safer alternatives.
Sustainability: Elemental analysis helps assess reliance on finite resources (mined minerals) and promotes sustainable sourcing and greener formulations.
the building blocks
The chemistry of pigments is fascinating but not easy to explain (at least for me!). Here are some basics from which you can explore further.
1. Atoms are the basic building block of all matter and chemistry. Atoms can combine with other atoms to form molecules but cannot be divided into smaller parts by ordinary chemical processes.
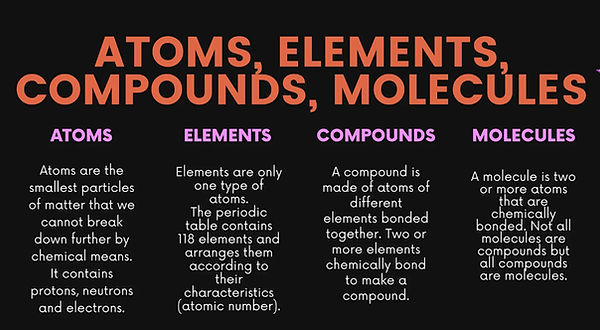
2. Elements are pure substance defined by having only one specific type of atom, distinguished by its unique number of protons (atomic number)Elements are the basic building blocks (atoms).
3, Molecules are combinations of atoms.
4. Compounds are a specific category of molecules where the atoms are all different elements.

The Periodic Chart above isn't intended to make you cross-eyed and give you a headache; it's an important tool to have when you want to understand your pigments better. Now, I cried everyday after chemistry (the whole 3 weeks of college chem I took before dropping out in frustration and dismay!), and swore I couldn't think of a single good reason I should learn any of this. Well, that came back to bite me when I started working with pigments and analyzing them to understand their natures better. This isn't to say I know anything about chemistry, but I've found that knowing vermilion is derived from mercury, I at least know not to lick it or use it as a cosmetic.
An element's position and properties (including the electron configuration) determine color, stability, and behaviors of each pigment. The periodic table is key to understanding mineral pigments because an element's position and properties (especially electron configuration) directly determine the color, stability, and chemical behavior of the resulting pigment.
The primary color-producing elements are transition metals, located in the central d-block of the table. So, iron is the primary element and the secondary element is manganese, the secondary element will influence the overall color. A perfect example of a three-element mineral compound is Vivianite. With just iron, phosphorus and a bit of hydrogen, the color of the base element, iron, is altered due to the phosphorus to blue. Another example of color influencing is when manganese is combined with iron to produce deep browns. With the manganese content ranging from 5%-20%, the higher the content the darker the brown and separates it from other iron oxide compounds.
But, additionally the manganese offers other benefits like a faster drying time when mixed with an oil binder. It also makes the colors cooler, slightly more green and ranging into darker hues. It's the amount of manganese that determines the shade and depth of umber color. Compare umbers to siennas and we find siennas carry more iron oxide and less manganese making the colors warmer and lighter but ranging from yellow to reddish brown.
5% to 20% manganese oxides (e.g., manganese dioxide, MnO₂) is what gives umber its distinctive dark brown color and sets it apart from yellow ochre.
Many times its that secondary (or trace) element that affects behaviors like drying time, lightfastness and other behaviors more than the primary element does.
I have come to rely on my periodic table so much I have one hanging over my desk. I refer to it often when I want to delve into the "DNA" of a pigment, see who it's related to, how it will behave, and where it'll play nicely.
So, if you're as curious and nosy as I am, you'll come to regard your periodic table as a valuable tool!

