pigment primer
are dyes and pigments really different materials?
It can be confusing trying to figure out the distinctions between dyes, which must have pigments to provide coloration, and the word “pigment” we use to refer to the actual colorants. There is clearly a lack of adequate language to make it clear to which type of pigment we’re referring, so you’ll have to take it on faith that when the word “pigment” is used, particularly here at PRI, we mean inorganic materials that include minerals, crystals, stones, clays and earths. There are also organic pigments which are derived from plants and animals and are most commonly used as dyes, but can be laked to create a paint pigment, and synthetic pigments which are man-made from chemicals.
Below you’ll find a lot more information about the differences between dyes and pigments, just in case you ever have to give testimony about it in court. The difference between dye and pigments involves chemistry and application methods and affects durability and other factors such as film strength, lightfastness and permanence.
Dyes
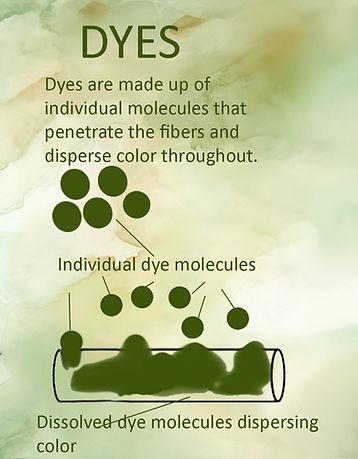
Dye molecules are extremely small (and in fact are individual molecules whereas pigments are composed of multiple molecules) and easily dissolve in water and other solvents which helps them bond with fibers at the molecular level. Some dyes require a mordant, or binder, to help them bond with the fibers.
Unlike pigments, dyes are organic and are made up of carbon rings and chains. They must be extracted from plants, insects, and in some cases, animals such as a particular species of sea snail from which Tyrian Purple is made. Another, much newer class of organic pigments are those being derived from microbes. This new group of pigments are opening up a lot of valuable potential in the pigment world.
When a dye is applied to a textile, it penetrates the fibers and settles evenly throughout (if the textile is wet; if it's dry the dye molecules don’t flow and penetrate as well and make blotches). Each molecule of dye bonds at the molecular level with the fibers. Even though organic pigments tend to be brighter, transparent and the colors more vibrant than inorganic pigments, they are more sensitive to light and heat. They are prone to fading and even when fixed with a mordant to the textile, they can still washout over time.
If a dye is applied to a substrate such as wood, it acts as a stain, permeating the wood fibers the same way it permeates the fibers of a textile. However, staining power is weak at best and is not permanent; the stain can be soaked out of the wood.
Dyes can be turned into paint through the process called “laking”. They do not create a film on the surface of a substrate, but soak in and bond molecularly with the material.


One of the steps in the laking process. photo courtesy Lucy Mayes, London Pigment
Pigments
The first thing to understand about pigments, whether earth or mineral, is they each have a unique elemental composition. For example, red ochre is Fe2 O3, (also called: ferrous oxide, iron oxide) and is the base formula for all iron oxides. Compare that with the formula for celadonite: (K(Mg,Fe^{2+})(Fe^{3+},Al)[Si_{4}O_{10}](OH)_{2}\). It is a potassium, magnesium, iron, aluminum silicate hydroxide in which the magnesium and iron can be found in variable proportions.
A pigment’s physical properties play a vital role in it’s performance. Particle size, particle shape and distribution, and refractivity all affect the color, opacity/transparency, finish and durability.
All pigments require a binder to make them adhere to the substrate. Unlike dyes, when mixed with a binder, pigments remain in suspension and create a film on the surface of the substrate but do not bond with it.
Pigments are particulates of soil, clay, stone or minerals that are composed of multiple molecules bonded together and are inorganic in nature meaning they are earth or mineral in origin if natural. Synthetic pigments are also inorganic and chemically modeled after earth and mineral pigments.
Earth pigments are naturally occurring minerals, soils, clay and stones and have proven the test of time in cave paintings and rock art worldwide. They include ochres, siennas, umbers, as well as green earth (celadonite, glauconite, chlorite), and chalk.
Another category of inorganic pigments is mineral pigments which are crushed rocks, minerals, and metal oxides like hematite, and magnetite.
Inorganic pigments have a low tinting strength which mutes the color/s they produce to earthy, natural colors. Both earth and mineral pigments are very stable; they resist fading from light and weathering giving them longevity. With a high pigment load and good refractivity because they have larger particles than dye, they have good opacity and coverage. In addition, they dry fast leaving a soft, matte finish that has a velvety texture.
Another category of inorganic pigments is mineral pigments which are crushed rocks, minerals, and metal oxides like hematite, and magnetite. Inorganic pigments have a low tinting strength which mutes the color/s they produce to earthy, natural colors. Mineral pigments share all the same characteristics as earth pigments but some are less stable chemically; some of the elements of which they are composed react to other elements and environmental factors in various ways.
It's always helpful to know what kind of pigment you're working with and from it's made. For example: azurite will alter chemically over time becoming malachite and changing from blue to green, particularly in humid environments. And the more you grind azurite the more color you lose due to reducing its refractive index with smaller particles.
Cinnabar/vermilion blackens over time, and vivianite goes through a range of colors from start to finish due to light sensitivity.
If you know from what a pigment is made elementally, the type of geological environment in which it formed, and have other information about it's characteristics, you can better use it to its full and best potential and avoid unpleasant surprises down the line.

Array of earth pigments. Click for image source.
Mineral pigments. Click for image source
Detail of the Virgin's robe where vermilion has darkened significantly. Click for image source
The last category of pigments falls into the inorganic section; synthetic pigments, while manmade are chemical replicas of mineral and earth pigments. They are designed to produce brighter, more stable colors, extend the range of colors, consistency in color, high tinting strength and good color saturation. They are also predictable as opposed to earth and mineral pigments which, because they are natural materials, have characteristics and behaviors we can't predict or completely control.



